Abstract
Irritable bowel syndrome (IBS) is the most prevalent disorder of brain-gut interactions that affects between 5 and 10% of the general population worldwide. The current symptom criteria restrict the diagnosis to recurrent abdominal pain associated with altered bowel habits, but the majority of patients also report non-painful abdominal discomfort, associated psychiatric conditions (anxiety and depression), as well as other visceral and somatic pain-related symptoms. For decades, IBS was considered an intestinal motility disorder, and more recently a gut disorder. However, based on an extensive body of reported information about central, peripheral mechanisms and genetic factors involved in the pathophysiology of IBS symptoms, a comprehensive disease model of brain-gut-microbiome interactions has emerged, which can explain altered bowel habits, chronic abdominal pain, and psychiatric comorbidities. In this review, we will first describe novel insights into several key components of brain-gut microbiome interactions, starting with reported alterations in the gut connectome and enteric nervous system, and a list of distinct functional and structural brain signatures, and comparing them to the proposed brain alterations in anxiety disorders. We will then point out the emerging correlations between the brain networks with the genomic, gastrointestinal, immune, and gut microbiome-related parameters. We will incorporate this new information into a systems-based disease model of IBS. Finally, we will discuss the implications of such a model for the improved understanding of the disorder and the development of more effective treatment approaches in the future.
Similar content being viewed by others
Introduction
IBS is one of the most common disorders of brain-gut interaction globally, with prevalence rates between 1.1 and 45% worldwide, and between 5 and 10% for most Western countries and China [1]. In contrast to many chronic non-communicable diseases, such as metabolic, neurological, cardiovascular and some forms of cancer, there has been no progressive increase in prevalence during the past 75 years, even though prevalence numbers have been fluctuating due to the periodic changes in official symptom criteria. Based on questionnaire data, women are 1.5–3.0 times more likely to have IBS, reflecting a prevalence in women of 14% and in men of 8.9% [2, 3]. However, based on healthcare system utilization, women are up to 2–2.5 times more likely to see a healthcare provider for their symptoms [4]. Based on the current symptom criteria [5], IBS is defined by chronically recurring abdominal pain associated with altered bowel habits in the absence of detectable organic disease. IBS symptoms can be debilitating in a small number of patients, but are mild to moderate in the majority of affected individuals [6]. Based on this definition, other frequently associated somatic or visceral pain and discomfort, as well as anxiety and depression are considered so called comorbid conditions.
The gut-restricted definition of the Rome criteria overlooks the fact that a large number of individuals who meet diagnostic criteria for an anxiety or depressive disorder have IBS and vice versa [7,8,9,10], and a majority of IBS patients show elevated levels of trait anxiety and neuroticism [10,11,12,13], or meet diagnostic criteria for an anxiety disorder [14]. Currently, the commonly associated psychiatric and somatic symptoms are generally referred to as comorbidities, separate from the primary GI diagnosis [15] and not present in all patients. However, detailed patient histories, frequently reveal symptoms of abdominal discomfort, anxiety and behavioral disturbances starting in early childhood in a majority of patients, and a large recent genetic epidemiological study has provided an intriguing explanation for the co-occurrence of abdominal and psychiatric symptoms in IBS patients on the basis of several shared single nucleotide polymorphisms (see paragraph IBS related genes shared with anxiety disorders below) [8]. These new findings are consistent with genetic vulnerabilities affecting both the central and the enteric nervous system (ENS), and argue against the long held linear pathophysiological concepts that emotional factors may cause IBS symptoms, or that chronic IBS gut symptoms lead to anxiety and depression Box 1.
Much of research and drug development in IBS patients has been based on descriptive and symptomatic features, rather than on biology-based disease definitions. These definitions suggest a core abnormality shared by all IBS patients (chronic, recurrent abdominal pain) as well as heterogeneity based on self reports of predominant bowel habit. However, a comprehensive identification of distinct biology-based subgroups of patients including those based on sex, with different underlying pathophysiological components and differential responsiveness to specific therapies, has not been achieved. Subtypes based on bowel habits are generally based on subjective reports of altered bowel habits, without consistent correlates in intestinal transit times, altered regional motility patterns or altered fluid and electrolyte handling by the gut [16]. Even though some of the most commonly used pharmacological and behavioral therapies are targeted at the level of the brain (low dose tricyclic antidepressants [17], serotonin reuptake inhibitors [18], cognitive behavioral therapies [19, 20], gut directed hypnosis, stress management [21]), research and drug development efforts are still predominantly focused on single, usually peripheral targets identified in preclinical models [16].
Based on such studies and on clinical reports from small samples, an astonishing list of biological abnormalities at various levels of the brain gut axis have been reported in the last 30 years and proposed as potential biomarkers or pathophysiological factors [2]: smooth muscle cells [22, 23], the gut epithelium [24]; bile acids [25,26,27,28]; immune system activation [29, 30]; neuroendocrine mechanisms [31]; brain structure and function [32, 33]; stress responsiveness [34]; affective [35, 36], cognitive [37,38,39,40], pain modulation [41, 42], gene polymorphisms [8]; and most recently the gut microbiome [43,44,45,46,47]. In addition, there has been a wealth of comprehensive data and clinical reports demonstrating a strong relationship between psychosocial factors and IBS symptoms [48]. However, despite the emergent discoveries about possible peripheral [29, 30] and central [32, 33, 35, 49, 50] components in IBS pathophysiology, the development of animal models with high face and construct validity [51], the reproduction of visceral hypersensitivity and IBS-relevant features after transplantation of human biospecimen into rodent models, and the recent acceptance of a brain-gut model of IBS [52], the controversy on the primary role of the nervous system versus peripheral factors still persists in the field [33, 53].
In this review, we will discuss the evidence supporting an integrative brain gut microbiome (BGM) model (Fig. 1) which incorporates a large body of evidence from studies on peripheral and central neurobiological disease mechanisms, brain and gut targeted influences of the exposome, and results from recently reported large scale genetic analyses with relevance for neuronal dysfunction of the CNS (central nervous system) and ENS (enteric nervous system). This systems biological model is consistent with the frequent comorbidity of IBS with other so-called functional GI disorders, and with other chronic pain and psychiatric disorders, in particular with anxiety. We will use this model to discuss the implications for the pathophysiology of IBS, its association with psychiatric symptoms, and the development of more effective treatment approaches in the future.
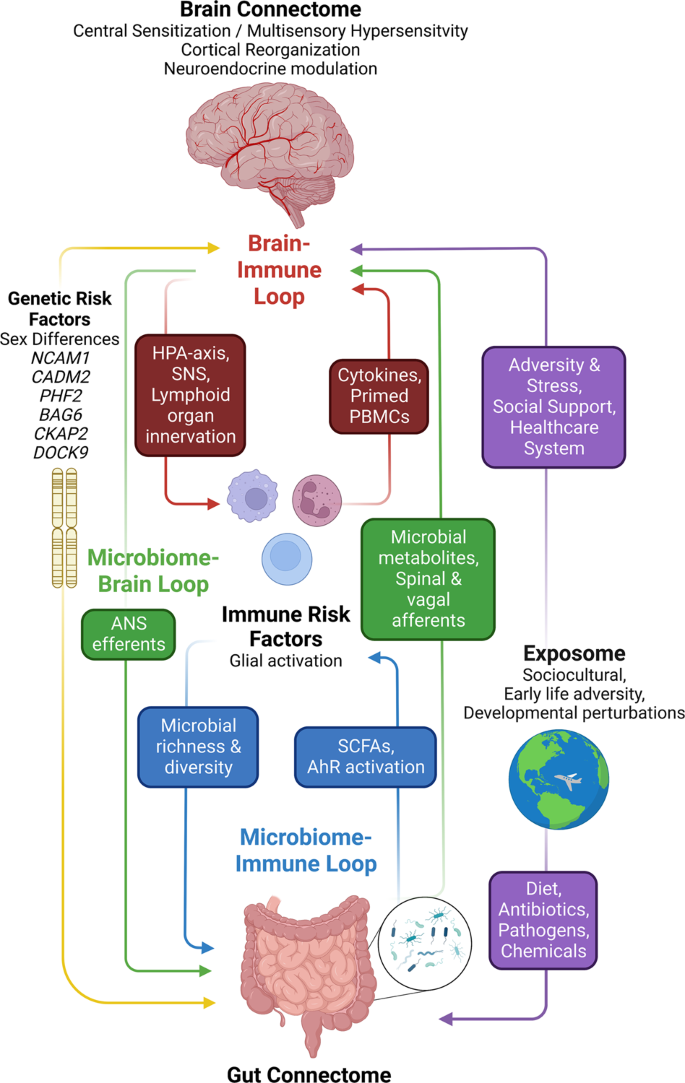
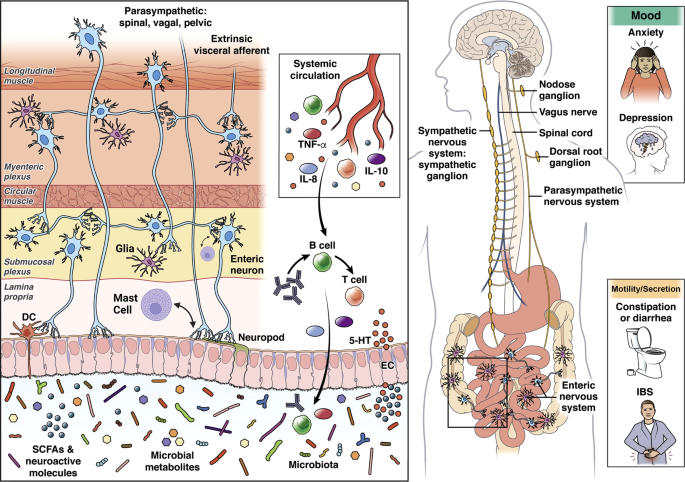
The brain connectome, gut connectome and gut microbiome communicate in a bidirectional way. The response characteristics of the system are determined by vulnerability genes interacting with different influences from the exposome. The different loops use neural, endocrine, paracrine and immune signaling mechanisms. Perturbations (stressors) of the different nodes of the system (brain, gut, immune, microbiota) result in non-linear effects and alterations in response characteristics manifesting as psychiatric and/or gut symptoms. ANS autonomic nervous system, SNS sympathetic nervous system, PBMCs peripheral blood mononuclear cells, SCFAs short chain fatty acids, AhR aryl hydrocarbon receptor.
The brain-gut-microbiome system
The enteric nervous system and gut connectome
The ENS is a vast network of different types of intrinsic enteric neurons and glia which are “sandwiched” between the mucosa, and the circular and longitudinal muscle layers of the gut, containing motor neurons, intrinsic primary afferent neurons, and interneurons. Nearly every neurotransmitter class found in the CNS is present in the ENS [54]. These neurons are organized into two interconnected networks, the myenteric and submucosal plexus, which regulate motility and secretion respectively in a coordinated fashion [55]. Different classes of neurons are chemically coded by different combinations of neurotransmitters and modulators, many of which are also found in the CNS [56].
Within the gut, the ENS is closely connected with the gut-based immune system, endocrine system, glial and epithelial cells, making up the gut connectome [57] (Fig. 2). The term connectome reflects close proximity, connectivity, and functional interactions between many cell types and functions in the gut that interact with ENS and CNS.
Alterations in these interactions can present as psychiatric and/or IBS symptoms. Modified with permission from [79].
Beyond the gut, the ENS is connected with the spinal cord, brainstem, and brain via primary spinal and vagal afferents, and postganglionic sympathetic and vagal efferent fibers [58, 59]. Although the ENS is capable of regulating all GI functions without input from the CNS, the CNS (brain and spinal cord) has strong modulatory functions in regulating intestinal behaviors [60] in accordance with the overall state of the organisms and homeostatic perturbations [53].
Even though the ENS is often being referred to as the “second brain” [61], evolutionarily speaking, the ENS can be traced back to the cnidaria phylum and epitomized by the hydra genus 650 million years ago [62]. Historically, it has been classified as a nerve net, but evidence has shown specialized neurons with neurotransmitters such as serotonin, catecholamines, and neuropeptides are also involved [63, 64]. In the hydra, the main function of the ENS is peristalsis, mixing movements and expulsion in addition to avoidance behaviors, [62]. The process of cephalization and the development of bilateria (i.e., organisms through evolution with a head/tail [anterior/posterior axis] and belly/back [dorsal/ventral axis]) led to the development of more complex neuronal systems, most notably the CNS around a central region and highly developed brains. Thus from an evolutionary standpoint, the ENS can be considered “the first brain” [56, 62].
ENS related genes
A recent profiling of the human ENS at single-cell resolution highlighted important genes related to neuropathic, inflammatory, and extraintestinal diseases [65]. Overlapping with the largest GWAS of IBS to date [8], CADM2, encoding the cell-adhesion molecule, was highly expressed in myenteric but not mucosal glia [65]. The known functions of myenteric glia include modulating myenteric neuron activity, regulating oxidative stress and neuroinflammation, providing trophic support, gliogenesis, and neurogenesis [66]. CADM2 encodes a member of synaptic cell adhesion molecules (SynCAMs) involved in synaptic organization and signaling [67], and cell adhesion-mediated mechanisms underlying the communication between glia and neurons in the ENS are important in understanding of ENS function in health and disease. For example, perturbed communication between enteric glia and neurons may play a role in dysfunctional ENS circuits in IBS [66]. The mechanisms underlying neuronal-glia signaling of the ENS in the context of gastrointestinal disorders, IBS, and visceral pain has recently been extensively reviewed [66, 68]. It is worth noting that CADM2 has been implicated in a wide range of psychological and neurological traits often observed in IBS patient including, but not limited to psycho-behavioral traits, risk-taking behavior, nervousness-like traits, and neurodevelopmental disorders (e.g., intellectual disability and autism spectrum disorder) [69]. Moreover, SynCAMs have a large role in synaptogenesis, axon guidance, and synaptic plasticity at a basic neurodevelopmental level which has the potential to affect a variety of disorders [70].
Similarly, NCAM1 is another gene found in the largest GWAS to date and has been implicated in the development of the ENS. In a similar manner to CADM2, NCAM1 has been shown to play a role in the ENS regarding cell migration, axon growth, neuronal plasticity and fasciculation [71], but has not been as thoroughly investigated as CADM2. A recent cross-tissue atlas applied single-nucleus RNA sequencing from eight healthy human organs showed that a cluster of genes including NCAM1 and CADM2 were involved particularly with cognitive/psychiatric symptoms including general cognitive ability, risk-taking behavior, intelligence, and neuroticism [72]. Even though the study did not contain tissue samples from the intestinal regions of the ENS, these genes involved in cognitive/psychiatric functions were highly expressed in Schwann cells in the esophagus mucosa, and interstitial cells of Cajal (ICCs) and neurons in the esophagus muscularis [72].
The gut microbiome
The term gut microbiome refers to the 40 trillion microbial organisms (bacteria, fungi, and archae) and their millions of genes that live throughout the gastrointestinal tract, from the oral cavity to the rectum, with the highest concentration and diversity in the large bowel [73]. The symbiotic interactions of the 3 groups of microorganisms within the microbiome, and with the extensive gut virome are incompletely understood [74, 75]. The characterization of these microorganisms in IBS to date is primarily based on identification of relative abundances and diversity using 16S rRNA sequencing techniques with limited resolution beyond the species level. We refer to several recent review articles on this topic [76, 77]. The extensive literature reveals inconsistent findings and a causative relationship of specific microorganisms with IBS symptoms has not been demonstrated. However, both preclinical and some clinical studies have demonstrated a significant effect of psychosocial stress on the relative abundance of gut microbes which is mediated both by stress-induced alterations in regional transit and secretion, and by direct effects of norepinephrine and possibly other signaling molecules released from gut cells on gut microbial gene expression and virulence [78], suggesting the possibility that the microbiome in subgroups of IBS patients with greater stress reactivity may contribute to certain symptoms [79].
Brain Connectome alterations in IBS
A growing body of research paired with clinical observations supports a critical role of the brain in the generation and maintenance of IBS symptoms. Regardless of primary symptom triggers, the brain is ultimately responsible for constructing and generating the conscious perception of abdominal pain, discomfort, and anxiety based on sensory input from the gut. Stressful and traumatic events during early life increase chances of developing IBS, and psychosocial stressors in adulthood play a crucial role during the first onset, symptom flare, and perceived severity of the symptoms [80]; centrally targeted pharmacological treatments and cognitive behavioral strategies have been some of the most effective IBS treatment strategies [3, 16, 81].
Specific brain functions such as sensory processing and modulation, emotion regulation, or cognition are the result of dynamic interactions of distributed brain areas operating in large-scale networks. As summarized in Fig. 3C and Table 1, these central networks and their properties have been assessed by neuroanatomical and neurophysiological studies in animals [51], as well as by a wealth of studies using different structural and functional brain imaging techniques and analyses in humans [82,83,84,85,86].
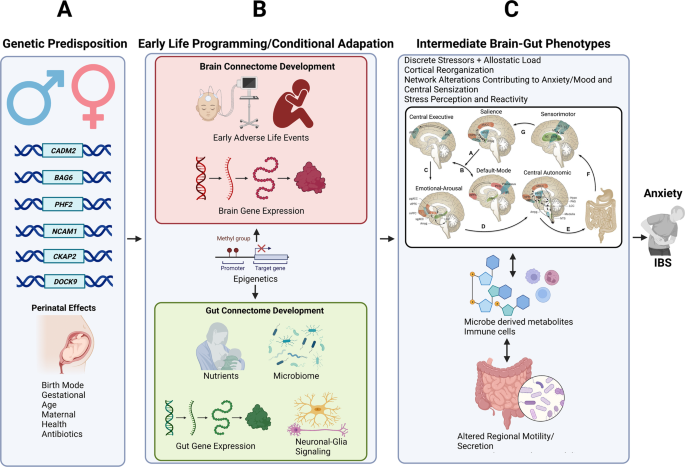
a Vulnerability genes and prenatal influences (including maternal health, nutrition, and stress level) on BGM system development. b the brain and gut transcriptome is influenced by mode of delivery, early adversity, and early nutrition, leading to the development of distinct intermediate brain gut phenotypes c which shape the adult response to influences from the exposome (diet, psychosocial stress).
In humans, several types of networks have been reported [33] (summarized in Table 1): functional brain networks based on evoked responses [87] or intrinsic connectivity of the brain during rest [82, 83]; structural networks based on gray matter parameters [88] and white matter properties; and anatomical networks based on white matter connectivities [89]. Both evoked and resting state studies performed in patients with IBS have demonstrated abnormalities in regions and task-related networks linked to salience detection [90, 91], emotional arousal [92,93,94,95], central autonomic control [38, 96,97,98], central executive control [90, 94, 99], and sensorimotor processing [38, 100, 101]. IBS-related alterations in these networks have provided plausible neurobiological substrates for several information-processing abnormalities reported in patients with IBS, such as stress hyperresponsiveness, biased threat appraisal, expectancy of outcomes, cognitive inflexibility, autonomic hyperarousal (emotional arousal and central autonomic networks), symptom-focused attention (central executive network) [33, 53] and cognitive inflexibility (central executive network). Supporting the concept of shared pathophysiological factors (so called p-factors), several reported brain network alterations have also been described in other chronic pain conditions [102] and in anxiety disorders (see Table 1).
The Salience Network
The salience network (SN) is integral in mediating the switching of activation between the default mode network (DMN) and central executive network, coordinating and adjusting physiologic/behavioral responses to internal and environmental perturbations of homeostasis [103]. Visceral inputs to the affective-motivational component of the SN converge onto the anterior insula coordinating response selection and conflict monitoring with the dACC [103]. Controlled rectal distention in IBS subjects has been shown consistently to result in increased engagement of the core hubs of the SN which are associated with increased affective, emotional, and arousal processes [104,105,106]. Reduced neurokinin-1 receptor (NK-1R) availability in the dACC, reflecting NK-1R endocytosis in response to substance P release, was found to be associated with duration of IBS symptoms [107]. Increased substance P release is thought to result from noxious visceral stimuli and increased engagement of endogenous pain or stress inhibition systems [107]. In adolescent girls with IBS, lower gray matter volume of the dACC has been observed [108], and greater salience-sensorimotor connectivity quantified by multiple neuroimaging techniques predicts a lack of symptom alleviation over 3–12 months in patients with IBS [109].
The default mode network (DMN)
The DMN’s role in pain perception is known to act as an opposite manner to the SN, such that the DMN is suppressed when attention is placed on present sensory stimuli, and is activated when attention is engaged with thoughts away from present sensory stimuli and engaged in mind wandering (i.e., thoughts unrelated to the present sensory environment) [110]. Studies in chronic pain subjects have shown altered functional connectivity and topological reorganization in various regions, consistent with DMN dysregulation [111]. Overall neuroimaging research suggests decreased activity of the DMN in patients with IBS [112]. Lower integrity of anatomical connectivity and resting-state functional connectivity, and lower morphological integrity within the DMN (between the aMPFC and PCC) were found to be predictive of sustained IBS symptom severity over 3–12 months [109]. Rectal lidocaine administration in IBS subjects was associated with decreased pain perception and with increased coherence in the DMN [113], supporting an involvement of the DMN in visceral hypersensitivity in patients with IBS.
The Sensorimotor Network
Similar to other chronic pain disorders, imaging studies in IBS subjects have shown alterations of the sensorimotor network (SMN), consistent with alterations in central processing and modulation of viscerosensory and somatosensory information [32, 100, 109, 114,115,116,117]. This network consists of the primary motor cortex, area 24 of the cingulate cortex, premotor cortex, supplementary motor area (SMA), posterior operculum/insula, as well as primary and sensory cortices in the parietal lobe. In addition, lower gray matter volume in the basal ganglia and thalamus as well as greater functional connectivity within the SMN have been observed in young children with chronic pain [108]. Greater intrinsic functional connectivity in adults, greater cortical thickness of the posterior insula positively associated with symptom duration, and increasing functional coupling of area 24 and the thalamus, and greater SMN connectivity to the SN predicting sustained symptoms over 3–12 months [109]. When viewed together, current evidence suggests patients with IBS have functional, morphological, and microstructural SMN alteration, which are likely to play a role in the increased perception of both visceral and somatic stimuli.
The central autonomic network
The central autonomic network (CAN) regulates visceromotor, neuroendocrine, pain, and behavioral responses essential for survival [118]. Afferents project through the spinal cord and eventually arrive at the main homeostatic processing sites in the brainstem/central autonomic network (including hypothalamus, amygdala, and PAG), and higher cortical processing and modulatory regions [119]. Historically it has been difficult to non-invasively study the brain stem nuclei in humans due to the limited spatial resolution of neuroimaging methods, but new imaging protocols with a resolution of 1mm3 and below are allowing new insights [120].
The CAN is closely connected by vagal and sympathetic efferent projections with the ENS, and afferents from the ENS send viscerosensory signals back to the brain. The hubs of the SN also participate in autonomic control via descending projections to the amygdala (tagging emotional valence and engaging autonomic survival responses to behaviorally relevant stimuli), hypothalamus (regulating homeostasis and a pattern generator for the stress response) and brainstem structures including the periaqueductal gray (PAG) and locus coeruleus (LC). The PAG is a key structure for integrating autonomic, pain modulatory/analgesic, and motor responses to stress [121], and the LC-norepinephrine system plays a central role in behavioral arousal and stress responses [122,123,124].
When viewed together, based on a large number of structural, and functional (resting state and evoked) studies, IBS patients show alterations in several brain networks related to salience assessment, attention, stress perception and responsiveness, and sensory processing. The responsiveness and connectivity of these networks are modulated by several vulnerability genes, which are shared both with ENS genes, and with genes identified in anxiety disorders. Based on these findings, we hypothesize that perturbations of homeostasis arising from the exposome, in the form of psychosocial and gut-targeted stressors interact with genetic factors to a spectrum of clinical phenotypes, ranging from gut symptoms to anxiety.
IBS-related genes shared with anxiety disorders
Prior to the availability of biobank scale data, many candidate gene studies uncovered potential pathways underlying IBS symptoms. These pathways have been extensively reviewed and include the serotonin pathway, SCN5A, and intestinal channelopathy, and sucrase-isomaltase malabsorption [125]. As serotonin is secreted from enteroendocrine cells and activates enteric sensory and motor neurons, expression level alterations in serotonin receptors and transporters are likely to play a potential role in visceral hypersensitivity, pain, intestinal motility, and secretion. SCN5A encodes the voltage-gated sodium Nav1.5 channel present on interstitial cells of Cajal (ICCs) in the ENS [31, 126]. Genetic mutations on this gene have shown to impair peristalsis and cause constipation, even though slow transit constipation is an uncommon finding in IBS-C [127]. Lastly, two faulty copies of the SI gene result in reduced disaccharide activity responsible for degradation of sucrose and starch, resulting in diarrhea and gas production in the large intestine from bacterial fermentation and is termed congenital sucrase-isomaltase deficiency (CSID), and should not be considered as IBS [125]. Even though these findings have established causal relationships between specific genetic abnormalities and non-specific IBS-like GI symptoms in a small number of affected individuals, it is highly unlikely that they play an important role in the great majority of patients.
Recently, the largest genome wide association study with 53,000 cases of IBS across multiple cohorts was completed [8]. In this study, the strongest risk factors for IBS included long-term or recurring antibiotic exposure in childhood, somatic pain conditions (back pain, limb pain, headaches), psychiatric conditions (anxiety, depression, excessive worrying) and fatigue. The genes included CADM2, BAG6, PHF2/FAM120AOS, NCAM1, CKAP2/TPTE2P3, and DOCK9. Four of the six loci are highly implicated in anxiety/mood disorders and there was a strong genome-wide genetic correlation of IBS with anxiety, neuroticism, depression, insomnia, and schizophrenia. Moreover, the high genetic correlations persisted after taking into account individuals with phenotypic overlap, suggesting common etiological pathways between IBS and anxiety/mood disorders. Implication of the central nervous system was further suggested by the finding that the six identified loci regulate gene expression in many genes primarily expressed in the brain. As already mentioned under ENS above, the genes NCAM1 and CADM2 were two genes which regulate neural circuit formation and influence changes in white matter microstructure in IBS and mood disorders [128,129,130]. Specifically, they regulate synaptic cell adhesion molecules, which are present in dorsal root ganglia sensory neurons throughout development, mediate adhesion of sensory axons, and induce neurite outgrowth [130]. Mechanisms relating to brain development were further implicated by the genes PHF2 (i.e., proper expansion of neural progenitors) and DOCK9 (i.e., dendritic development of the hippocampus), but have not yet been studied in patients with IBS [131,132,133].
Importantly, the heritability of IBS was estimated to be a modest 5.8%, suggesting that perturbation of the brain-gut axis by environmental factors arising from the exposome such as early adversity, psychosocial stress, learned behaviors, diet, and possibly dysbiosis play a prominent role.
Considering these new genetic findings and the reported frequent comorbidities of IBS with other chronic pain and psychiatric conditions it is becoming increasingly recognized that IBS is part of a constellation of symptoms that occur on a larger spectrum of altered brain-body interactions [134, 135]. This concept is consistent with the “somatic symptom disorder” concept, previously proposed [2]. The main co-occurring symptoms include hypersensitivity to multiple internal and external sensory stimuli, which could explain the observed association with a variety of seemingly unrelated external and internal factors, previously reported. Other co-occurring symptoms include mood problems, fatigue, and problems with sleep onset and maintenance, as well as memory disturbance [134]. The neurogenetic basis integrating mood/anxiety and central amplification of sensory inputs (“central sensitization”) based on many of these genetic hits have been well established, which will be discussed below.
Known functions of NCAM1, DOCK9, and PHF2 and possible roles in IBS pathophysiology are summarized in Table 2.
Central sensitization and comorbid chronic pain conditions
The primary mechanism for the core symptom of persistent, chronically recurring abdominal pain that patients with IBS report is thought to result from alterations in the central processing of sensory input from the gut, also referred to as central sensitization [134, 136]. The term was originally coined to represent the specific spinal mechanisms responsible for the amplification of nociceptive signaling involving spinal activation of the NMDA receptor [137, 138], and is present in various chronic pain disorders such as chronic neuropathic pain, fibromyalgia, headaches, and IBS [6, 134, 139,140,141]. Today, it is understood that spinal and supraspinal mechanisms both play key roles in the development and maintenance of central sensitization. Based on rodent models of pain, plausible spinal mechanisms include alterations in converging sensory input from different sites on the GI tract and body, temporal and spatial summation, reduced endogenous dorsal horn inhibition, and glial cell activation. Based on human brain imaging studies, supraspinal mechanisms include an altered balance between facilitatory and inhibitory endogenous pain modulation influences, hyperconnectivity between brain networks, alterations of gray matter architecture, elevated CSF glutamate and substance P levels, reduced GABAergic transmission, altered noradrenergic signaling/receptors, and glial cell activation [122, 134].
The large overlap - up to a 4.27 odds ratio - between psychiatric phenotypes (primarily anxiety and depression [136, 142]) and IBS and other chronic pain disorders, as well as genetic overlap [8, 143,144,145] mentioned earlier, suggests central sensitization as a possible shared pathophysiological factor (p factor) [134, 146,147,148]. The concept of central sensitization was introduced in psychological research in the 1990s based on the observation that highly sensitive persons (HSPs) often share a history of early adversity, psychological profile of introversion (“neuroticism”), and greater emotionality [149]. Patients with IBS are significantly more likely to exhibit qualities of HSPs, and show central sensitization which is expressed as general sensory hypersensitivity [150]. The association between chronic pain disorders, psychiatric symptoms, and mechanisms of central sensitization is likely due to the above-mentioned supraspinal alterations, including monoamine neurotransmitter systems (i.e., serotonin, dopamine, noradrenaline), the amino acid GABA, and brain regions underlying both pain transmission/modulation and mood disorders [151, 152]. Striato-thalamic-frontal cortical pathways including the prefrontal cortex, amygdala, nucleus accumbens, and thalamic nuclei are key hubs, and alterations in neuronal firing and communication underlie sensory sensitivity and psychiatric symptoms including altered perception, arousal, cognition, and mood [152,153,154]. Behaviorally, chronification of central sensitization and negative mood states have been proposed to be in the same continuum of aversion, such that pain motivates the avoidance of further injury, and anxiety promotes behaviors that diminish anticipated danger [154].
An extensive literature supports the importance of early programming by early adverse life (EAL) events for the development not only of IBS [76], but also of other chronic pain conditions and psychiatric syndromes [155, 156]. Perturbations to the developing brain play a large sole in sensitizing cortical nociceptive circuitry [157], with the most mechanistic study in humans showing larger event-related potentials (ERPs) to nociceptive stimuli, but not tactical stimuli in infants exposed to many invasive, skin-breaking, painful procedures and morphine [158]. Moreover, up to 68.4% of children who are exposed to early life traumatic events such as the NICU can develop chronic pain by age 10. Greater amounts of pain-related stressors, painful procedures, and morphine are associated with lower global gray matter volumes throughout childhood [159, 160]. In addition to the well documented changes in stress response systems [161,162,163], the effect of early-life dietary influences on the gut microbiome and the BGM axis have received increasing attention, even though a direct link with chronic abdominal pain has not been established [164, 165].
Clinical and therapeutic implications
Despite a decades-long effort by the pharmaceutical industry, a large number of IBS candidate drugs identified and validated in preclinical models and targeted at both central and gut mechanisms have failed, either due to lack of efficacy or serious side effects [16]. Of the small number of new drugs obtaining FDA approval, efficacy above placebo has generally not exceeded 10% in phase 3 trials. The great majority of available, FDA approved IBS medications are targeted at intestinal secretion and motility, and the gut microbiome with the goal to improve altered bowel habits and bloating-type symptoms in subgroups of patients [16].
Pharmacological treatments have been clinically divided into first and second-line approaches [16], and are aimed at specific symptoms. Moderate quality data has shown low-dose tricyclic antidepressants and SSRIs to be effective for pain (primarily the former) and comorbid anxiety and depression (primarily the latter) [16, 18]. As 5-HT receptor-mediated signaling plays important roles both in the brain, as well as in the gut, there is a good rationale for IBS treatments targeted at these receptors. 5-HT released from enterochromaffin cells mediates many GI functions including peristalsis, secretion, pain, and nausea via receptors on ENS and vagal nerve endings [31]. For example, 5HT-3 receptor antagonists (acting on both gut and brain-located 5HT-3 receptors (such as alosteron, and ramosteron) have shown effectiveness in slowing colonic transit, improving diarrhea, and reducing visceral pain in well-designed randomized controlled trials [16]. High-quality preclinical data has shown the antagonism of 5HT-3 receptors on the area postrema and vagus nerve have shown a reduction of visceral pain and diarrhea [16, 18, 166], and older data have demonstrated anxiolytic effects [167,168,169].
Despite evidence obtained in rodent models of IBS, efforts to develop peripheral visceral analgesics or central stress modulators (antagonists for CRF-1 and NK-1 receptors) have failed to show therapeutic benefits in IBS. This is surprising, as multiple preclinical studies as well as a human brain imaging study had demonstrated effectiveness of the CRF-R1 antagonist Emicerfont (GW876008) on evoked visceral pain and on central stress circuits [170, 171]. Because of these disappointing results, increased attention has been shifted to behavioral treatments, including gut-directed hypnosis [21, 81, 172,173,174,175], mindfulness-based stress reduction [176], and cognitive behavioral approaches [19, 20, 177,178,179]. Several of these therapeutic approaches have shown promise in improving IBS symptoms, and a few studies have demonstrated associated neurobiological effects on brain mechanisms in salience, emotional arousal, and executive networks [172, 177].
As access to therapists specialized in these behavioral IBS treatments is limited, and traditional delivery is time-consuming, web-based versions of these therapies have been evaluated, some of which have been FDA approved and are becoming available to patients [180]. In addition, several randomized controlled studies have shown some benefits of certain dietary interventions (low FODMAP diet [16]), and microbiome-targeted treatments (probiotics, antibiotics) [181].
Summary and conclusions
Even though in subsets of patients, SSRIs and bowel movement targeted therapies are helpful, the model of IBS presented in this review provides precedence for a multidisciplinary therapeutic approach including pharmacological, behavioral, and dietary approaches. Current evidence suggests that there are significant interindividual variations in the response to such therapies, including the predominant bowel habit subtype, severity of gut and psychiatric symptoms, and possibly the presence of gut microbial alterations.
There is growing evidence from clinical, preclinical, and genetic studies supporting the existence of shared p factors in IBS and often comorbid gastrointestinal and non-gastrointestinal pain conditions, as well as psychiatric conditions. Despite shared vulnerability genes, different influences from the environment (exposome) in particular during childhood ultimately shape the specific clinical phenotype. The emerging disease model can explain the failure of reductionistic single mechanism targeted treatment approaches, and is consistent with the evidence for the effectiveness of personalized multidisciplinary approaches involving behavioral, dietary, and pharmacological interventions.
Glossary
irritable bowel syndrome (IBS); brain-gut-microbiome (BGM); gastrointestinal (GI); enteric nervous system (ENS); central nervous system (CNS); synaptic cell adhesion molecules (SynCAMs); default mode network (DMN); salience network (SAL); sensorimotornNetwork (SMN); central autonomic network (CAN); central executive network (CEN); locus coeruleus (LC); periaqueductal grey (PAG); dorsal anterior cingulate cortex (dACC); posterior cingulate cortex (PCC); N-methyl-D-aspartate (NMDA); gamma-aminobutyric acid (GABA); cerebrospinal fluid (CSF); early adverse life events (EAL); serotonin (5-HT); selective serotonin reuptake inhibitor (SSRI); long-term potentiation (LTP); event-related potentials (ERPs).
References
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21.
Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Prim. 2016;2:16014.
Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet 2020;396:1675–88.
Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Grant Thompson W, et al. U. S. Householder survey of functional gastrointestinal disorders. Dig Dis Sci. 1993;38:1569–80.
Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016;150:1262–79.
Simrén M, Törnblom H, Palsson OS, Van Oudenhove L, Whitehead WE, Tack J. Cumulative effects of psychologic distress, visceral hypersensitivity, and abnormal transit on patient-reported outcomes in irritable bowel Syndrome. Gastroenterology 2019;157:391–402.e2.
Banerjee A, Sarkhel S, Sarkar R, Dhali GK. Anxiety and depression in Irritable Bowel Syndrome. Indian J Psychol Med. 2017;39:741–5.
Eijsbouts C, Zheng T, Kennedy NA, Bonfiglio F, Anderson CA, Moutsianas L, et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat Genet. 2021;53:1543–52.
Bengtson M-B, Aamodt G, Vatn MH, Harris JR. Co-occurrence of IBS and symptoms of anxiety or depression, among Norwegian twins, is influenced by both heredity and intrauterine growth. BMC Gastroenterol. 2015;15:9.
Lee C, Doo E, Choi JM, Jang S-H, Ryu H-S, Lee JY, et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J Neurogastroenterol Motil. 2017;23:349–62.
Muscatello MRA, Bruno A, Mento C, Pandolfo G, Zoccali RA. Personality traits and emotional patterns in irritable bowel syndrome. World J Gastroenterol. 2016;22:6402–15.
Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–60.
Hu Z, Li M, Yao L, Wang Y, Wang E, Yuan J, et al. The level and prevalence of depression and anxiety among patients with different subtypes of irritable bowel syndrome: a network meta-analysis. BMC Gastroenterol. 2021;21:23.
Fadgyas-Stanculete M, Buga A-M, Popa-Wagner A, Dumitrascu DL. The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J Mol Psychiatry. 2014;2:4.
Mayer EA, Catherine Bushnell M. Functional Pain Syndromes: Presentation and Pathophysiology. Lippincott Williams & Wilkins; 2015.
Camilleri M. Diagnosis and treatment of irritable bowel syndrome: a review. J Am Med Assoc. 2021;325:865–77.
Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–53.
Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EMM, Moayyedi P, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut–brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:117–31.
Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol Res Behav Manag. 2017;10:231–7.
Lackner JM, Jaccard J, Keefer L, Brenner DM, Firth RS, Gudleski GD, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel Syndrome. Gastroenterology 2018;155:47–57.
Flik CE, Bakker L, Laan W, van Rood YR, Smout AJPM, de Wit NJ. Systematic review: The placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol. 2017;23:2223–33.
Takeshita E, Matsuura B, Dong M, Miller LJ, Matsui H, Onji M. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol. 2006;41:223–30.
Miller LJ. Characterization of cholecystokinin receptors on human gastric smooth muscle tumors. Am J Physiol. 1984;247:G402–G410.
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189.
Wei W, Wang H-F, Zhang Y, Zhang Y-L, Niu B-Y, Yao S-K. Altered metabolism of bile acids correlates with clinical parameters and the gut microbiota in patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol. 2020;26:7153–72.
Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol. 2018;16:522–7.
Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharm Ther. 2015;42:3–11.
Bajor A, Törnblom H, Rudling M, Ung K-A, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015;64:84–92.
Hughes PA, Zola H, Penttila IA, Blackshaw LA, Andrews JM, Krumbiegel D. Immune activation in irritable bowel syndrome: can neuroimmune interactions explain symptoms? Am J Gastroenterol. 2013;108:1066–74.
Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–73.
Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions, and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–86.
Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain 2015;156:S50–S63.
Mayer EA, Labus J, Aziz Q, Tracey I, Kilpatrick L, Elsenbruch S, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut 2019;68:1701–15.
Larauche M, Mulak A, Taché Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67.
Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25:386–94.
Elsenbruch S, Schmid J, Bäsler M, Cesko E, Schedlowski M, Benson S. How positive and negative expectations shape the experience of visceral pain: an experimental pilot study in healthy women. Neurogastroenterol Motil. 2012;24:914–e460.
Aizawa E, Sato Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology 2012;143:1188–98.
Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain 2013;154(Suppl 1):S63–S70.
Kennedy PJ, Clarke G, O’Neill A, Groeger JA, Quigley EMM, Shanahan F, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychol Med. 2014;44:1553–66.
Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–9.
Piché M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010;148:49–58.
Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011;60:1589–99.
Ringel Y, Ringel-Kulka T. The intestinal microbiota and Irritable Bowel Syndrome. J Clin Gastroenterol. 2015;49(Suppl 1):S56–S59.
Ringel Y. The gut microbiome in irritable bowel syndrome and other functional bowel disorders. Gastroenterol Clin North Am. 2017;46:91–101.
Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–23.e8.
Zhuang X, Xiong L, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38.
Bennet SMP, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318–31.
Lackner JM. The role of psychosocial factors in gastrointestinal disorders. Gut. 2014;33:104–16.
Grundy L, Erickson A, Brierley SM. Visceral Pain. Annu Rev Physiol. 2019;81:261–84.
Al Omran Y, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol. 2014;11:565–76.
Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of visceral pain: pathophysiology, translational relevance and challenges/B. Am J Physiol Gastrointest Liver Physiol. 2015;463:G885–G903.
Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology 2022;162:300–15.
Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592–605.
Furness JB, Callaghan BP, Rivera LR, Cho H-J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71.
Furness JB The Enteric Nervous System. London, England: Blackwell Publishing; 2005.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–94.
Bohórquez DV, Liddle RA. The gut connectome: making sense of what you eat. J Clin Invest. 2015;125:888–90.
Margolis KG, Gershon MD, Bogunovic M. Cellular organization of neuroimmune interactions in the gastrointestinal tract. Trends Immunol. 2016;37:487–501.
Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13:517–28.
Browning KN, Alberto, Travagli R. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes. 2019;26:11–6.
Gershon M. The Second Brain: The Scientific Basis of Gut Instinct and a groundbreaking new understanding of nervous disorders of the stomach and intestine. HarperCollins; 1998.
Furness JB, Stebbing MJ. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil. 2018;30. https://doi.org/10.1111/nmo.13234.
Kass-Simon G, Pierobon P. Cnidarian chemical neurotransmission, an updated overview. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:9–25.
Westfall JA, Elliott SR, MohanKumar PS, Carlin RW. Immunocytochemical evidence for biogenic amines and immunogold labeling of serotonergic synapses in tentacles of Aiptasia pallida (Cnidaria, Anthozoa). Invertebr Biol. 2005;119:370–8.
Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, et al. The human and mouse enteric nervous system at single-cell resolution. Cell 2020;182:1606–22.e23.
Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571–87.
Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 2002;297:1525–31.
Morales-Soto W, Gulbransen BD. Enteric Glia: A new player in abdominal pain. Cell Mol Gastroenterol Hepatol. 2019;7:433–45.
Pasman JA, Chen Z, Smit DJA, Vink JM, Van Den Oever MC, Pattij T, et al. The CADM2 gene and behavior: a phenome-wide scan in UK-Biobank. Behav Genet 2022;52:306–14. https://doi.org/10.1007/s10519-022-10109-8. 22 July 2022.
Frei JA, Stoeckli ET. SynCAMs – From axon guidance to neurodevelopmental disorders. Mol Cell Neurosci. 2017;81:41–8.
Fu M, Vohra BPS, Wind D, Heuckeroth RO. BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncam1 polysialic acid addition. Dev Biol. 2006;299:137–50.
Eraslan G, Drokhlyansky E, Anand S, Fiskin E, Subramanian A, Slyper M, et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science 2022;376:eabl4290.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut 2022;71:1020–32.
Liang G, Bushman FD. The human virome: assembly, composition and host interactions. Nat Rev Microbiol. 2021;19:514–27.
Cao Z, Sugimura N, Burgermeister E, Ebert MP, Zuo T, Lan P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022;81:104113.
Osadchiy V, Martin CR, Mayer EA. The Gut-brain axis and the microbiome: mechanisms and clinical implications. Clin Gastroenterol Hepatol. 2019;17:322–32.
Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–48.
Sandrini S, Aldriwesh M, Alruways M. Microbial endocrinology: host–bacteria communication within the gut microbiome. J Endocrinol 2015;225:R21–R34.
Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 2021;160:1486–501.
Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 2000;47:861–9.
Ford AC, Quigley E, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–65.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH, et al. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. NeuroImage 2012;61:1471–83.
Bullmore E, Sporns O. The economy of brain network organization. Nat Neurosci Rev. 2012;13:336–49.
Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage 2017;160:15–31.
Sporns O, Betzel RF. Modular brain networks. Annu Rev Psychol. 2016;67:613–40.
Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, et al. Brain imaging approaches to the study of functional GI disorders: A Rome Working Team Report. Neurogastroenterol Motil. 2009;21:579–96.
Tijms BM P, Series, Willshaw DJ, Lawrie SM. Similarity-based extraction of individual networks from gray matter MRI Scans. Cereb Cortex. 2012;22:1530–41.
Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE. 2007;2:e597.
Gupta A, Kilpatrick L, Labus J, Tillisch K, Braun A, Hong J-Y, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76:404–12.
Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology 2006;131:352–65.
Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2012;10:385–90.
Dickhaus B, Mayer EA, Firooz N, Stains J, Conde F, Olivas TI, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. 2003;98:135–43.
Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain 2013;154:2088–99.
Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100.
Farmer AD, Aziz Q. Visceral pain hypersensitivity in functional gastrointestinal disorders. Br Med Bull. 2009;91:123–36.
Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–63.
Tillisch K. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut 2005;54:1396–401.
Hong J-Y, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–9.
Hong J-Y, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-Mcnalley C, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33:11994–2002.
Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, et al. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS). Biol Psychol. 2010;84:272–8.
Martucci KT, MacKey SC. Neuroimaging of pain: human evidence and clinical relevance of central nervous system processes and modulation. Anesthesiology 2018;128:1241–54.
Menon V. Salience network. Brain Mapp: Encycl Ref. 2015;2:597–611.
Hall GBC, Kamath MV, Collins S, Ganguli S, Spaziani R, Miranda KL, et al. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22:276–e80.
Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology 2010;139:1310–9.
Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut 2010;59:489–95.
Jarcho JM, Feier NA, Bert A, Labus JA, Lee M, Stains J, et al. Diminished neurokinin-1 receptor availability in patients with two forms of chronic visceral pain. Pain 2013;154:987–96.
Bhatt RR, Gupta A, Labus JS, Zeltzer LK, Tsao JC, Shulman RJ, et al. Altered Brain Structure and Functional Connectivity and Its Relation to Pain Perception in Girls With Irritable Bowel Syndrome. Psychosom Med. 2019;81:146–54.
Bhatt RR, Gupta A, Labus JS, Liu C, Vora PP, Jean S, et al. A neuropsychosocial signature predicts longitudinal symptom changes in women with irritable bowel syndrome. Mol Psychiatry. 2022;27:1774–91.
Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95.
Qi R, Ke J, Joseph Schoepf U, Varga-Szemes A, Milliken CM, Liu C, et al. Topological reorganization of the default mode network in irritable bowel syndrome. Mol Neurobiol. 2016;53:6585–93.
Nisticò V, Rossi RE, D’Arrigo AM, Priori A, Gambini O, Demartini B. Functional neuroimaging in irritable bowel syndrome: a systematic review highlights common brain alterations with functional movement disorders. J Neurogastroenterol Motil. 2022;28:185–203.
Letzen JE, Craggs JG, Perlstein WM, Price DD, Robinson ME. Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J Pain. 2013;14:1077–87.
Ellingson BM, Mayer E, Harris RJ, Ashe-Mcnally C, Naliboff BD, Labus JS, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain 2013;154:1528–41.
Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS ONE. 2013;8:e73932.
Piché M, Chen JI, Roy M, Poitras P, Bouin M, Rainville P. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain. 2013;14:1217–26.
Labus J, Dinov I, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, et al. Irritable Bowel Syndrome in female patients is associated with alterations in structural brain networks. Pain 2014;155:137–49.
Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:998–1001.
Lamotte G, Shouman K, Benarroch EE. Stress and central autonomic network. Auton Neurosci. 2021;235:102870.
Napadow V, Sclocco R, Henderson LA. Brainstem neuroimaging of nociception and pain circuitries. PAIN Rep. 2019;4:e745.
Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–89.
Suárez-Pereira I, Llorca-Torralba M, Bravo L, Camarena-Delgado C, Soriano-Mas C, Berrocoso E. The role of the Locus Coeruleus in pain and associated stress-related disorders. Biol Psychiatry. 2022;91:786–97.
Valentino RJ, Van, Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharm. 2008;583:194–203.
Taché Y, Mönnikes H, Bonaz B, Rivier J. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann N. Y Acad Sci. 1993;697:233–43.
Camilleri M, Zhernakova A, Bozzarelli I, D’Amato M. Genetics of irritable bowel syndrome: shifting gear via biobank-scale studies. Nat Rev Gastroenterol Hepatol. 2022;19:689–702.
Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907.
Beyder A, Mazzone A, Strege PR, Tester DJ, Saito YA, Bernard CE, et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology 2014;146:1659–68.
Petrovska J, Coynel D, Fastenrath M, Milnik A, Auschra B, Egli T, et al. The NCAM1 gene set is linked to depressive symptoms and their brain structural correlates in healthy individuals. J Psychiatr Res. 2017;91:116–23.
Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein Kinase c and the RAS–mitogen-activated protein kinase pathway. J Neurosci. 2000;20:2238–46.
Frei JA, Andermatt I, Gesemann M, Stoeckli ET. The SynCAM synaptic cell adhesion molecules are involved in sensory axon pathfinding by regulating axon-axon contacts. Development 2015;142:e0106–e0106.
Kuramoto K, Negishi M, Katoh H. Regulation of dendrite growth by the Cdc42 activator Zizimin1/Dock9 in hippocampal neurons. J Neurosci Res. 2009;87:1794–805.
Pappa S, Padilla N, Iacobucci S, Vicioso M, Álvarez de la Campa E, Navarro C, et al. PHF2 histone demethylase prevents DNA damage and genome instability by controlling cell cycle progression of neural progenitors. Proc Natl Acad Sci USA. 2019;116:19464–73.
Shi L. Dock protein family in brain development and neurological disease. Commun Integr Biol. 2013;6:e26839.
Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021;397:2098–110.
Nijs J, George SZ, Clauw DJ, Fernández-de-las-Peñas C, Kosek E, Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol 2021;3:e383–e392.
Midenfjord I, Grinsvall C, Koj P, Carnerup I, Törnblom H, Simrén M. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol Motil. 2021;33:e14156.
Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686–8.
Latremoliere A, Woolf CJ. Central SENSITIZATION: A GENERATOR OF PAIN HYPERSENSITIVITY BY CENTRAL NEURAL PLASTicity. J Pain. 2009;10:895–926.
Verne NG, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain 2003;103:99–110.
Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–704.
Caldarella MP, Giamberardino MA, Sacco F, Affaitati G, Milano A, Lerza R, et al. Sensitivity disturbances in patients with irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2006;101:2782–9.
Iimura S, Takasugi S, Division R, Co M Hsp and gastrointestinal disease symptoms. https://psyarxiv.com/n2c39/download?format=pdf. Accessed 7 August 2022.
Mocci E, Ward K, Dorsey SG, Ament SA, GWAS meta-analysis reveals dual neuronal and immunological etiology for pain susceptibility. medRxiv. 2021:2021.08.23.21262510.
McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 2003;106:127–33.
Tang J, Gibson SJ. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain. 2005;6:612–9.
Clark JR, Nijs J, Yeowell G, Holmes P, Goodwin PC. Trait sensitivity, anxiety, and personality are predictive of central sensitization symptoms in patients with chronic low back pain. Pain Pr. 2019;19:800–10.
Shigetoh H, Tanaka Y, Koga M, Osumi M, Morioka S. The mediating effect of central sensitization on the relation between pain intensity and psychological factors: a cross-sectional study with mediation analysis. Pain Res Manag. 2019;2019:3916135.
Adams LM, Turk DC. Psychosocial factors and central sensitivity syndromes. Curr Rheumatol Rev. 2015;11:96–108.
Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol. 1997;73:345–68.
Boyce WT. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology 2016;41:142–62.
Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav. 2013;7:1–14.
Elman I, Borsook D. Threat response system: parallel brain processes in pain vis-à-vis fear and anxiety. Front Psychiatry. 2018;9:29.
Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci. 2015;282:20142516.
Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015;87:474–91.
Zouikr I, Bartholomeusz MD, Hodgson DM. Early life programming of pain: focus on neuroimmune to endocrine communication. J Transl Med. 2016;14:123.
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, Mccarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9.
Verriotis M, Chang P, Fitzgerald M, Fabrizi L. Development of the Nociceptive brain. Neuroscience 2016;338:207–19.
Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage 2010;52:583–9.
Van Den Bosch GE, White T, El Marroun H, Simons SHP, Van Der Lugt A, Van Der Geest JN, et al. Prematurity, Opioid exposure and neonatal pain: do they affect the developing brain? Neonatology 2015;108:8–15.
Ranger M, Chau CMY, Garg A, Woodward TS, Beg MF, Bjornson B, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013;8:e76702.
Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28.
Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39.
Lippard ETC, Nemeroff CB. The devastating clinical consequences of child abuse and neglect: increased disease vulnerability and poor treatment response in mood disorders. Am J Psychiatry. 2020;177:20–36.
Coley EJL, Hsiao EY. Malnutrition and the microbiome as modifiers of early neurodevelopment. Trends Neurosci. 2021;44:753–64.
Ratsika A, Codagnone MC, O’Mahony S, Stanton C, Cryan JF. Priming for life: early life nutrition and the microbiota-gut-brain axis. Nutrients 2021;13:423.
Andresen V, Montori VM, Keller J, West CP. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in non constipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Biomed Res. 2008;6:545–55.
Costall B, Naylor RJ. Anxiolytic potential of 5-HT3 receptor antagonists. Pharm Toxicol. 1992;70:157–62.
Fakhfouri G, Rahimian R, Dyhrfjeld-Johnsen J, Zirak MR, Beaulieu J-M. 5-HT3 receptor antagonists in neurologic and neuropsychiatric disorders: the iceberg still lies beneath the surface. Pharm Rev. 2019;71:383–412.
Olivier B, van Wijngaarden I, Soudijn W. 5-HT(3) receptor antagonists and anxiety; a preclinical and clinical review. Eur Neuropsychopharmacol. 2000;10:77–95.
Hubbard CS, Labus JS, Bueller J, Stains J, Suyenobu B, Dukes GE, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci. 2011;31:12491–500.
Labus JS, Hubbard CS, Bueller J, Ebrat B, Tillisch K, Chen M, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology 2013;145:1253–61.e3.
Lowén MBO, Mayer EA, Sjöberg M, Tillisch K, Naliboff B, Labus J, et al. Effect of hypnotherapy and educational intervention on brain response to visceral stimulusin the irritable bowel syndrome. Aliment Pharm Ther. 2013;37:1184–97.
Rutten JMTM, Reitsma JB, Vlieger AM, Benninga MA. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Child. 2013;98:252–7.
Rutten JMTM, Vlieger AM, Frankenhuis C, George EK, Groeneweg M, Norbruis OF, et al. Home-based hypnotherapy self-exercises vs individual hypnotherapy with a therapist for treatment of pediatric irritable bowel syndrome, functional abdominal pain, or functional abdominal pain syndrome. JAMA Pediatr. 2017;171:470.
Peters SL, Muir JG, Gibson PR. Review article: gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment Pharm Ther. 2015;41:1104–15.
Naliboff BD, Smith SR, Serpa JG, Laird KT, Stains J, Connolly LS, et al. Mindfulness-based stress reduction improves irritable bowel syndrome (IBS) symptoms via specific aspects of mindfulness. Neurogastroenterol Motil. 2020;32:e13828.
Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome 2021;9:236.
Lackner JM, Keefer L, Jaccard J, Firth R, Brenner D, Bratten J, et al. The Irritable Bowel Syndrome Outcome Study (IBSOS): Rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self-versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. 2012;33:1293–310.
Edebol-Carlman H, Ljótsson B, Linton SJ, Boersma K, Schrooten M, Repsilber D, et al. Face-to-face cognitive-behavioral therapy for irritable bowel syndrome: the effects on gastrointestinal and psychiatric symptoms. Gastroenterol Res Pr. 2017;2017:8915872 https://doi.org/10.1155/2017/8915872.
Owusu JT, Sibelli A, Moss-Morris R, van Tilburg MAL, Levy RL, Oser M. A pilot feasibility study of an unguided, internet-delivered cognitive behavioral therapy program for irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14108.
Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharm Ther. 2018;48:1044–60.
Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–8.
Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N. Y Acad Sci. 2008;1124:1–38.
Northoff G. Anxiety disorders and the brain’s resting state networks: from altered spatiotemporal synchronization to psychopathological symptoms. Adv Exp Med Biol. 2020;1191:71–90.
Kim Y-K, Yoon H-K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:115–22.
MacNamara A, DiGangi J, Phan KL. Aberrant spontaneous and task-dependent functional connections in the anxious brain. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:278–87.
Kolesar TA, Bilevicius E, Wilson AD, Kornelsen J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. NeuroImage: Clin. 2019;24:102016.
ten Donkelaar HJ, Broman J, van Domburg P The Somatosensory System. In: ten Donkelaar HJ, editor. Clinical Neuroanatomy: Brain Circuitry and Its Disorders, Cham: Springer International Publishing; 2020. p. 171–255.
Woodworth D, Mayer E, Leu K, Ashe-McNalley C, Naliboff BD, Labus JS, et al. Unique microstructural changes in the brain associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: A MAPP network neuroimaging study. PLoS One. 2015;10:e0140250.
Grinsvall C, Ryu HJ, Van Oudenhove L, Labus JS, Gupta A, Ljungberg M, et al. Association between pain sensitivity and gray matter properties in the sensorimotor network in women with irritable bowel syndrome. Neurogastroenterol Motil. 2020;33:e14027.
Bouziane I, Das M, Friston KJ, Caballero-Gaudes C, Ray D. Enhanced top-down sensorimotor processing in somatic anxiety. Transl Psychiatry. 2022;12:295.
Brandl F, Weise B, Mulej Bratec S, Jassim N, Hoffmann Ayala D, Bertram T, et al. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 2022;47:1071–80.
Berman SM, Chang L, Suyenobu B, Derbyshire SW, Stains J, FitzGerald L, et al. Condition-specific deactivation of brain regions by 5-HT3 receptor antagonist Alosetron. Gastroenterology 2002;123:969–77.
Tillisch K, Labus J, Nam B, Bueller J, Smith S, Suyenobu B, et al. Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with irritable bowel syndrome: a pilot study. Aliment Pharm Ther. 2012;35:360–7.
Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501.
Hong J-Y, Naliboff BD, Labus JS, Kilpatrick LA, Fling C, Ashe-McNalley C, et al. Sa2014 IBS patients show altered brain responses during uncertain, but not certain expectation of painful stimulation of the abdominal wall. Gastroenterology 2015;148:S–384.
Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–35.
Pessoa L. A network model of the emotional brain. Trends Cogn Sci. 2017;21:357–71.
Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage 2007;36:736–45.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34.
Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TOC, Evers EAT, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut 2011;60:1196–203.
Yágüez L, Coen S, Gregory LJ, Amaro E, Altman C, Brammer MJ, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology 2005;128:1819–29.
Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with Irritable Bowel Syndrome. J Neurosci. 2008;28:349–59.
Kilpatrick LA, Labus JS, Coveleskie K, Hammer C, Rappold G, Tillisch K, et al. The HTR3A Polymorphism c. -42C>T is associated with amygdala responsiveness in patients with irritable bowel syndrome. Gastroenterology. 2011;140:1943–51.
Harrewijn A, Cardinale EM, Groenewold NA, Bas-Hoogendam JM, Aghajani M, Hilbert K, et al. Cortical and subcortical brain structure in generalized anxiety disorder: findings from 28 research sites in the ENIGMA-Anxiety Working Group. Transl Psychiatry. 2021;11:502.
Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–8.
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42.
Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–68.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Afzal M, Potokar JP, Probert CSJ, Munafò MR. Selective processing of gastrointestinal symptom-related stimuli in irritable bowel syndrome. Psychosom Med. 2006;68:758–61.
Gibbs-Gallagher N, Palsson OS, Levy RL, Meyer K, Drossman DA, Whitehead WE. Selective recall of gastrointestinal-sensation words: evidence for a cognitive-behavioral contribution to irritable bowel syndrome. Am J Gastroenterol. 2001;96:1133–8.
Phillips K, Wright BJ, Kent S. Irritable bowel syndrome and symptom severity: Evidence of negative attention bias, diminished vigour, and autonomic dysregulation. J Psychosom Res. 2014;77:13–9.
Tkalcic M, Domijan D, Pletikosic S, Setic M, Hauser G. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol. 2014;38:621–8.
Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. NeuroImage 2009;47:952–60.
Seminowicz DA, Shpaner M, Keaser ML, Michael Krauthamer G, Mantegna J, Dumas JA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14:1573–84.
Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 2010;138:1783–9.
Qiu C, Liao W, Ding J, Feng Y, Zhu C, Nie X, et al. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res. 2011;194:47–53.
Naliboff BD, Berman S, Chang L, Derbyshire SWG, Suyenobu B, Vogt BA, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology 2003;124:1738–47.
Nagel M, Jansen PR, Stringer S, Watanabe K, De Leeuw CA, Bryois J, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet. 2018;50:920–7.
Ko H-G, Choi J-H, Park DI, Kang SJ, Lim C-S, Sim S-E, et al. Rapid turnover of Cortical NCAM1 regulates synaptic reorganization after peripheral nerve injury. Cell Rep. 2018;22:748–59.
Ao W, Cheng Y, Chen M, Wei F, Yang G, An Y, et al. Intrinsic brain abnormalities of irritable bowel syndrome with diarrhea: a preliminary resting-state functional magnetic resonance imaging study. BMC Med Imaging. 2021;21:4.
Chen A, Chen Y, Tang Y, Bao C, Cui Z, Xiao M, et al. Hippocampal AMPARs involve the central sensitization of rats with irritable bowel syndrome. Brain Behav. 2017;7:e00650.
Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66.
Kim H-J, Hur SW, Park JB, Seo J, Shin JJ, Kim S-Y, et al. Histone demethylase PHF2 activates CREB and promotes memory consolidation. EMBO Rep. 2019;20:e45907.
Wei F, Xu ZC, Qu Z, Milbrandt J, Zhuo M. Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation. J Cell Biol. 2000;149:1325–34.
Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–903.
Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom. 2014;13:397–406.
Lee J-G, Ye Y. Bag6/Bat3/Scythe: a novel chaperone activity with diverse regulatory functions in protein biogenesis and degradation. Bioessays 2013;35:377–85.
Kawahara H, Minami R, Yokota N. BAG6/BAT3: emerging roles in quality control for nascent polypeptides. J Biochem. 2013;153:147–60.
Binici J, Koch J. BAG-6, a jack of all trades in health and disease. Cell Mol Life Sci. 2014;71:1829–37.
Case CM, Sackett DL, Wangsa D, Karpova T, McNally JG, Ried T, et al. CKAP2 ensures chromosomal stability by maintaining the integrity of microtubule nucleation sites. PLoS One. 2013;8:e64575.
Zhang S, Wang Y, Chen S, Li J. Silencing of cytoskeleton-associated protein 2 represses cell proliferation and induces cell cycle arrest and cell apoptosis in osteosarcoma cells. Biomed Pharmacother. 2018;106:1396–403.
Author information
Authors and Affiliations
Contributions
EAM conceptualized the manuscript and played a major role in the writing process and critically reviewed the final manuscript. RRB did an extensive literature review, played a major role in the writing process, and critically reviewed the final manuscript. HJR did an extensive literature review, created the main table, and critically reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
EAM is a member of the scientific advisory boards of Danone, Axial Therapeutics, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mayer, E.A., Ryu, H.J. & Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol Psychiatry 28, 1451–1465 (2023). https://doi.org/10.1038/s41380-023-01972-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01972-w
This article is cited by
-
Neural circuits regulating visceral pain
Communications Biology (2024)
-
Non-invasive neuromodulation: an emerging intervention for visceral pain in gastrointestinal disorders
Bioelectronic Medicine (2023)
-
Evaluation of the diagnostic performance of estimated fecal calprotectin and serum intelectin-1 and C-reactive protein solo or in combination for differentiation between patients with query ulcerative colitis and irritable bowel syndrome
The Egyptian Journal of Internal Medicine (2023)
-
A CNS circuit that regulates gut motility
Nature Metabolism (2023)
-
Neuropathic Pain and Spinal Cord Injury: Management, Phenotypes, and Biomarkers
Drugs (2023)